Discussions regularly arise during training sessions about visible bare copper at the ends of component leads, for example. The soldering of printed circuit boards with an OSP finish (*), where the bare copper is still partly visible, also leads to comments more often. Remarks along the lines like how long will it function and the reliability in the future.
But are such comments justified? Where does this fear of bare copper in electronic products come from?
In part, this may be due to the old MIL-2000 standard, which stated that no bare copper should be visible. So if one dates back to the time when this was valid, it may have been imprinted that one had to ensure at all times that all ends of component leads should be covered with solder. So, after soldering and cutting off the lead ends, the soldering iron was diligently taken up again and the end was provided with some solder.
Nowadays, with the IPC-A-610 and IPC/J-STD-001 standards for solder joints, it is explicitly stated that copper visible on component cut ends is not a reason for rejection. And PCB lands where parts are still not covered by solder may also occur if the solder connection otherwise meets the requirements.
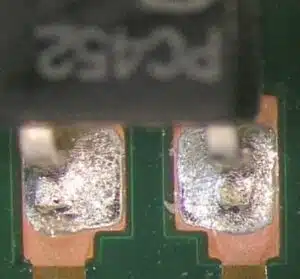
Copper visible, but solder joint meets the requirements.
And still there are operators who, if they see even the smallest piece of copper on an PCB land, resolutely reach for the soldering iron to “bury” it under a layer of solder. Sometimes even without being aware of the fact that by doing so they are actually deteriorating the connection. The argument that the exposed copper will oxidize is certainly true. It is important to know what influence the oxide layer that will form will have on the reliability of the product.
It is important in this context to know what oxidation actually means and that this should not be confused with corrosion. First, oxidation is a reaction process with oxygen (O2), corrosion is a process involving oxygen and water.
The oxide layer that forms on copper surfaces ensures that no corrosion can occur, this layer seals the underlying copper from the environment and therefore the underlying copper can no longer come into contact with oxygen.
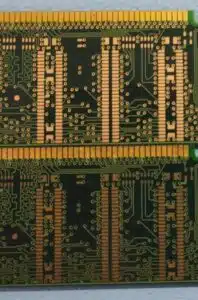
Printed circuit board with OSP finish (above) and after the OSP finish has been degraded and the copper has been oxidized (below).
The oxide layer protects the underlying copper and keeps this colour for years. Of course, the lower lands are no longer solderable.
The oxidation proceeds in stages, first of all the following reaction occurs: 4Cu+O2 leads to 2Cu2O, which will give a red/brown surface. Then it turns black because 2Cu2O + O2 decay into 4CuO (this process will be accelerated by heat). Then the typical patina formation of copper surfaces occurs (the green colour), but this can take years.

Green patina on a copper surface
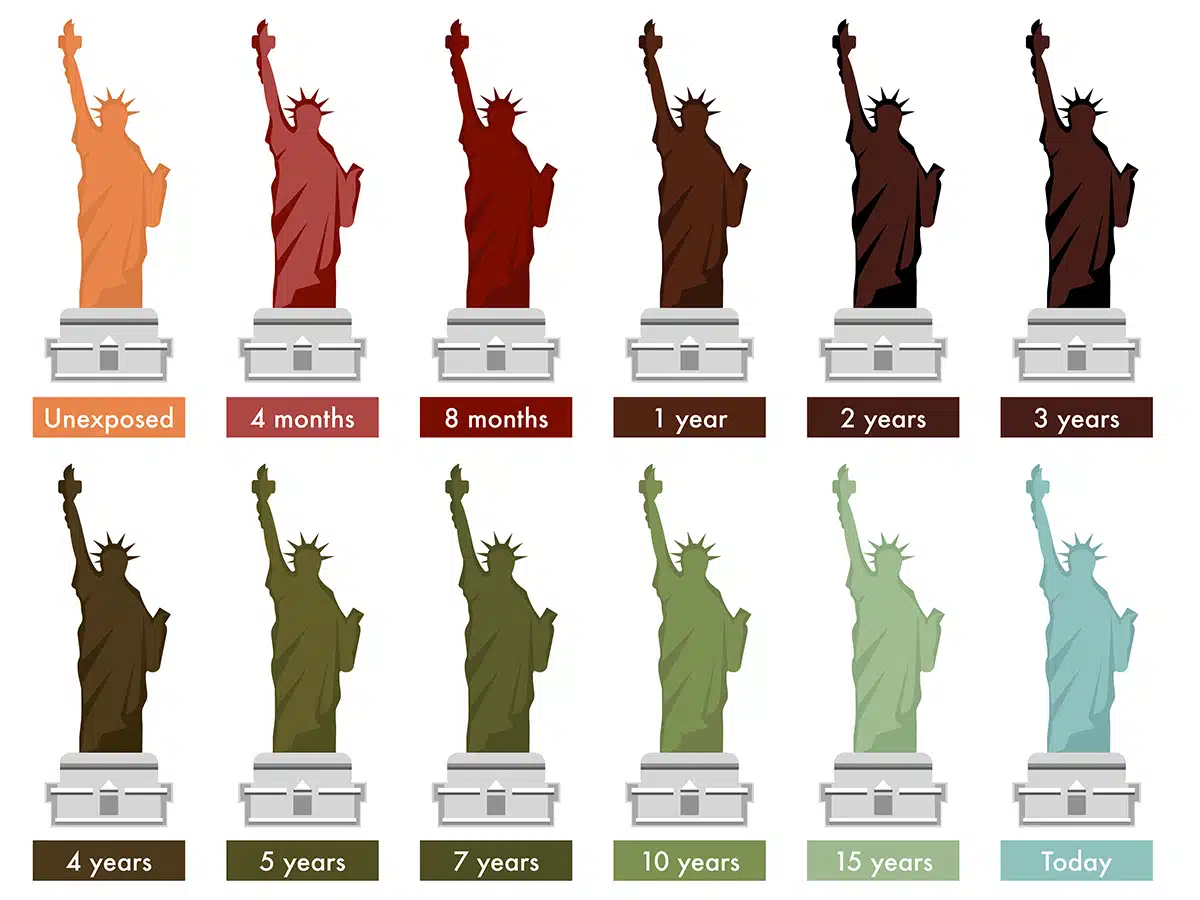
For years, the statue of liberty in the USA was shiny copper and red-brown, for example, before it turned green. If it had been regularly polished with copper gloss, it would still shine just as it did in 1886.
The green discoloration depends on various reactions, for example sulfur will have an influence on the final colour, but also CO, CO2 etc. The so-called brochantite, malachite or azurite reactions can then take place. In all cases the copper turns green, chemistry experts can even recognize which reactions have taken place by the color. For example, Cu2CO3(OH), Cu3(CO3)(OH)2 or Cu4So4(OH)6 can be formed, all green. The latter, also known as vitriol, finds applications in all kinds of areas, including keeping aquarium fish healthy. But other materials can also be used for this, such as simple charcoal, something that is also used in water tanks, for example, to keep the water quality optimal and to prevent algae formation.
| Colors of some copper compounds | |
| Cu | red |
| Cu2O | red to dark gray |
| FexCuyS | gold metallic |
| CuS | blue |
| Cu5FeS4 | gold brown to copper |
| Cu4SO4(OH)6 | green hydrated copper |
| Cu2Co3(OH)3 | green hydrated copper |
Most oxidation phenomena in copper can be negated by a hydrogen reaction, i.e. if you bring the product into a hydrogen-rich environment, the copper will regain its shine.
Processes in which surfaces are deliberately oxidized to protect the underlying metal are, for example, anodizing (where aluminum is electrolytically provided with an oxide layer on the surface, called eloxal) and galvanizing e.g. gutters, the zinc oxidizes on the surface and sacrifices itself so basically up for the underlying steel.
In the end, it may therefore be the case that people do not like the discoloration of the exposed copper in electronics, but this will not have a negative impact on operation or lifespan.
(*) OSP stands for Organic Solderability Preservative, which is an organic, clear coating that is sprayed directly onto the islands’ copper by the circuit board supplier and protects the copper.